Research Embedded Culture
Over the past three decades, researchers at the West Michigan Rheumatology have been at the forefront of clinical research in rheumatology. Our physicians and team have conducted a large number of investigations of new agents in rheumatic diseases.
We initiate our own studies and work with top tier academic colleagues in multicenter projects. We have broad experience from immunogenetic and proteomic descriptive studies, case control and cohort observation studies, as well as large scale survey, risk message framing experiments and phase 1-3 controlled clinical trials.
We have studied and published results in all of the conditions listed below. From decades of research experience we have developed deep knowledge and experience that enriches your care.
Scleroderma and Raynaud’s Phenomenon
Our research in scleroderma related diseases begin in 1989 with the recognition and description of the Eosinophilia Myalgia Syndrome and its relationship to epidemic Eosinophilic Fasciitis.

West Michigan Rheumatology was one of the early investigational sites for the Scleroderma Clinical Trials Consortium beginning in 1994 with the landmark “High dose, Low dose d-Penicillamine Trial”.
We have continued this thread of investigation by evaluating the validity of theRodnan Scleroderma Skin Score (a primary outcome in generalized scleroderma studies) and the Raynaud’s Condition Score (the primary outcome for all raynauds clinical trials). We have conducted a number of investigations of potential disease modifying treatments including “d-Penicillamine”, “relaxin” for generalized, diffuse scleroderma, “dasatimib” for pulmonary fibrosis in diffuse scleroderma, and “scleroderma treatment strategies”. In we a lead center in Cytori STAR Study. This evaluated the effectiveness of injection of autologously harvested fat stem cells into the fingers of patients with scleroderma related hand contractures. We also were investigators in the FocuSSCed Study. This evaluated subcutaneous Actemra (tocilizumab), an FDA approved treatment for RA, in early diffuse scleroderma. Positive results led to it to be the first FDA approved for the treatment of scleroderma related lung disease.
We have also participated in the design and conduct of several studies of potential therapies for Raynaud’s phenomenon: “oral iloprost“, “Vascana”, and “PF-00489791”. Our most recent study in this area is the AURORA Study which evaluated the effect of intravenous iloprost for symptomatic Raynaud’s in scleroderma.
Rheumatoid Arthritis
West Michigan Rheumatology has made a two decade commitment to advancing the care of patients with rheumatoid arthritis. We and citizens of West Michigan have contributed to the advancement of our knowledge and practice of caring for RA. Together we have participated in a long journey of observational studies of RA as well as investigations evaluating numerous therapeutic targets: Enbrel, Kineret, Remicade, Orencia, Actemra, Olumiant and Rinvoq as well as inhibitors of IL-1, CD-4, IL-15, IL-17, gamma interferon, CD20, and Blys. We have listed only a few of our publications which are linked their abstracts at the National Library of Medicine.
Timeline of FDA Approval of Disease Modifying Drugs for Rheumatoid Arthritis
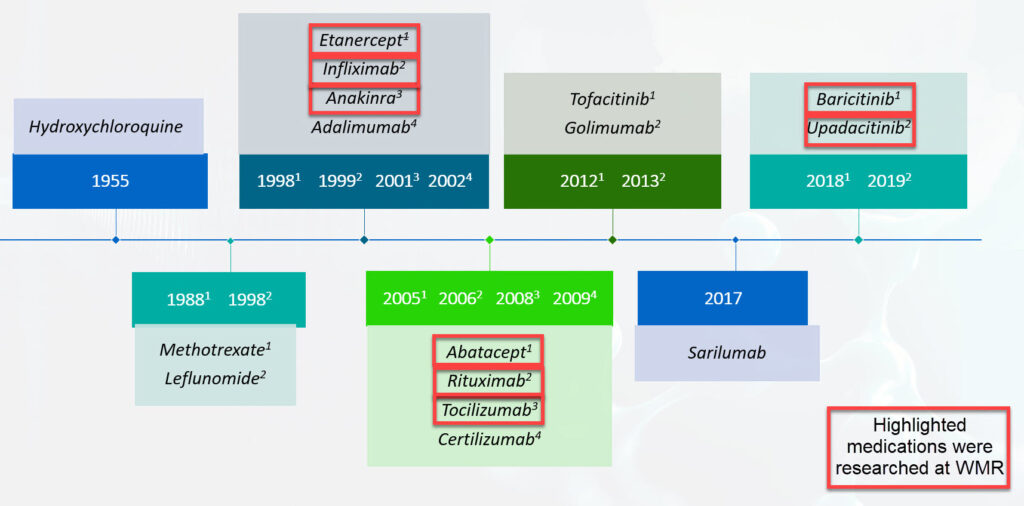
- A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Genovese MC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Finck BK. N Engl J Med. 2000;343:1586-93.
- Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Genovese MC, Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Cannon GW, Spencer-Green G, Finck BK. Arthritis Rheum. 2002 Jun;46(6):1443-50.
- Safety and efficacy of etanercept beyond 10 years of therapy in North American patients with early and longstanding rheumatoid arthritis. Weinblatt ME, Bathon JM, Kremer JM, Fleischmann RM, Schiff MH, Martin RW, Baumgartner SW, Park GS, Mancini EL, Genovese MC. Arthritis Care Res (Hoboken) 2010;63:373-82.
- A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Martin DA, Churchill M, Flores-Suarez L, Cardiel MH, Wallace D, Martin R, et al. Arthritis Res Ther. 2013;15:R164.
Psoriatic Arthritis
West Michigan Rheumatology was one of the original investigational sites for Enbrel – the first biologic approved for use in psoriatic arthritis. We also performed the early phase 1 and studies of Brodalumab (a monoclonal antibody directed against interleuken-17) in rheumatoid arthritis. Subsequently we conducted several clinical trials of its effectiveness and safety in patients with Psoriatic Arthritis. Our participation evaluating this novel therapeutic target is an extension of this series of investigations and shows our commitment to helping explore the possible conditions that could potentially benefit from targeted biologic therapy, including psoriatic arthritis.
TNF inhibition
- Mease PJ et al.J. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomized trial. Lancet. 2000 Jul 29;356(9227):385-90.
- Mease PJ et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004 Jul;50(7):2264-72.
- Pneumococcal vaccine response in psoriatic arthritis patients during treatment with etanercept. Mease PJ, Ritchlin CT, Martin RW, Gottlieb AB, Baumgartner SW, Burge DJ, Whitmore JB. J Rheumatol. 2004 Jul;31(7):1356-61.
IL-17 Inhibition
We were involved from the beginning providing input in the design and conduct of the original dose ranging trial of this class leading IL-17 inhibitor that is now approved as SILIQ.
- Martin DA, Churchill M, Martin R, Chung JB et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013 Oct 25;15(5):R164.
- Brodalumab in psoriatic arthritis: phase III AMVISION-1 and AMVISION-2 trials.
We have also developed, evaluated and nationally disseminated a novel Option Table Decision Aid for dermatologists and rheumatologists to use to support their conversations with patients with psoriatic arthritis considering a new immunomodulator. Check out our Patient Decision Aids to learn more.
Systemic Lupus Erythematosus
There remains a great unmet need for new effective and safe treatments for SLE. We have participated in a number of investigations of studies from preclinical, phase 1, 2 and 3 for three separate molecules designed to treat SLE. The search for more effective therapies for SLE continues. Our work together lead to the 2021 approval of Saphnelo (anifrolumab) for treatment moderate to severe systemic lupus erythematosus.
RSLV-132
- Doedens J, Jones W, Hill K, Mason M, Linsley P, Mease P, Dall’Era M, Aranow C, Martin R, Cohen S, Fleischmann R, Kivitz A, Burge D, Chaussabel D, Elkon K, Posada J, Gabel C. OP0186 Immune Complex Bound U1 and Y1 RNA Correlates with Interferon-Stimulated Gene Expression and Disease Activity: An Observational Study of Sysytemic Lupus Erythematosus Patients. Annals Rheum Dis 2015;75:S2: http://dx.doi.org/10.1136/annrheumdis-2016-eular.2747
- Doedens JR, Jones WD, Hill K, Mason MJ, Gersuk VH, Mease PJ, Dall’Era M, Aranow C, Martin RW, Cohen SB, Fleischmann RM, Kivitz AJ, Burge DJ, Chaussabel D, Elkon KB Posada JA. Blood-Borne RNA Correlates with Disease Activity and IFN-Stimulated Gene Expression in Systemic Lupus Erythematosus. J Immunol 2016;197: 2854-2863.
- Burge DJ, Eisenman J, Byrnes-Blake K, Smolak P, Lau K, Cohen SB, Kivitz AJ, Levin R, Martin RW, Sherrer Y, Posada JA. Safety, pharmacokinetics, and pharmacodynamics of RSLV-132, an RNase-Fc fusion protein in systemic lupus erythematosus: a randomized, double-blind, placebo-controlled study. Lupus 2017; 26(8):825-834.
Tabalumab
- Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: results from ILLUMINATE-1, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Isenberg DA, Petri M, Kalunian K, Tanaka Y, Urowitz MB, Hoffman RW et al. Ann Rheum Dis. 2016 Feb;75(2):323-31. doi: 10.1136/annrheumdis-2015-207653.
- Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Merrill JT, van Vollenhoven RF, Buyon JP, Furie RA, Stohl W, Morgan-Cox M et al. Ann Rheum Dis. 2016 Feb;75(2):332-40.
Anifrolumab
- Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Furie, R. A., Morand, E. F., Bruce, I. N., Manzi, S., Kalunian, K. C., Vital, E. M. et al. (2019). The Lancet Rheumatology. https://doi.org/10.1016/S2665-9913(19)30076-1
- Trial of Anifrolumab in Active Systemic Lupus Erythematosus. Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, Bae SC et al. N Engl J Med. 2020 Jan 16;382(3):211-221. doi: 10.1056/NEJMoa1912196. Epub 2019 Dec 18. PMID: 31851795.
Polymyositis and Dermatomyositis
This is another condition where there is a great unmet need for new treatments. We have participated in a phase 1b study of the anti-CD20 small modular immunopharmaceutical TRU-15 for polymyositis and dermatomyositis. In addition, Dr. Eggebeen, who has advanced training in myositis care at the University of Pittsburg, had done translational research linking myositis associated antibodies to the development of intersitial lung disease. Subsequently Dr. Eggebeen was a principle investigator in the National Institute of Health funded, first of it kind trial the “Rituximab in Myositis Study”.
Patient Decision Making & Decision Aids
To translate novel biologic therapeutics from the bench to the bedside, a recent thread of our RA research involves the assessment of literacy, factors that influence patient decision quality, patient skills to interpret risk, and the development of decision aids to support patient choice. We have published our work in a variety of peer reviewed journals including: J Rheumatology, Arthritis Rheumatism, Patient Education & Counseling and Medical Decision Making. In addition, our work has been promoted by Medscape.com, MyCME.com, The Rheumatologist as well as at numerous national academic meetings.
To learn more and see examples of types of decision supports go to our Patient Decision Aids page.